Dedicated subcutaneous administration device with secure needle protector

After industrialization of an initial version of the device, our R&D team has fully redesigned the new version based on competitive review of functionalities. In addition, we have co-developed and co-patented part of the product.
DEVELOPMENT PROCESS → PROJECT Concept Pre-study
→ OUTCOME Design History File
→ MANUFACTURING Injection Molding
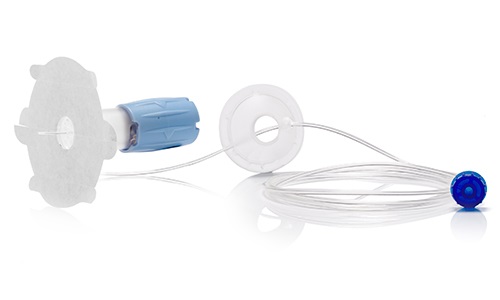
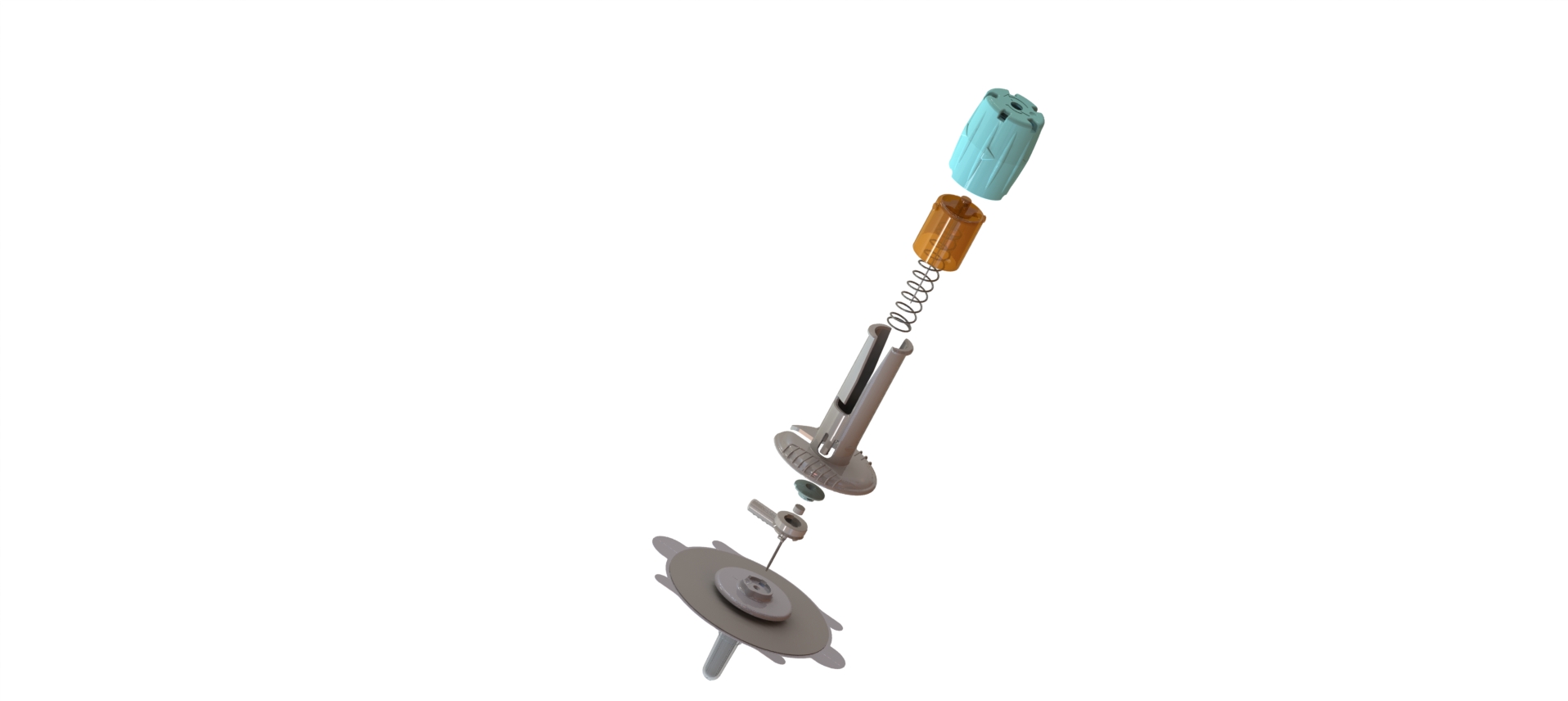
CAD Design
Rapid Prototyping
Material Selection
Design for Manufacture
Mold Manufacturing
Assembling Tools Manufacturing
FMEA Risk Analysis
IQ OQ PQ Validation Plan
CE Mark
FEP Tip Forming
Assembling
Packaging
Sterilization





