Thanks to our unique manufacturing expertise, our R&D team has helped our client, a contract manufacturer, to review and improve a critical production process manufacturing equipment.
DEVELOPMENT PROCESS → PROJECT Manufacturing Process Analysis
→ OUTCOME Design History File
This has resulted in improved capabilities and quality of products delivered.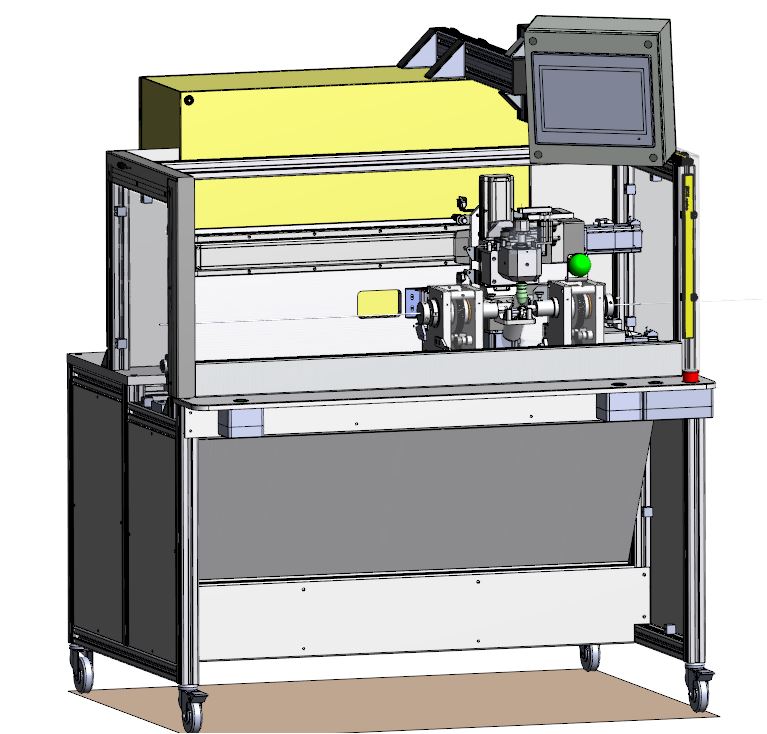
Mechanical CAD Design
Industrial Automatism Software Development
Equipment Manufacturing
FMEA Risk Analysis
IQ OQ PQ Validation Plan
CE Mark
CLASSIC LIST
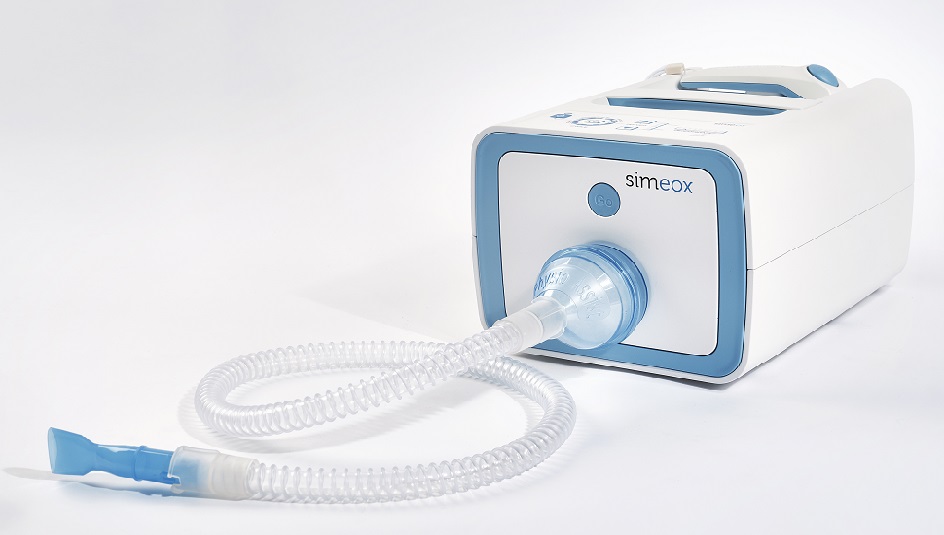
Our client, Physio-Assist has developed an innovative concept of equipment and tubing set dedicated to bronchial pathologies. We have acted as their product development and industrialization partner and have help drive the concept to fully operational equipment.
DEVELOPMENT PROCESS → EQUIPMENT PROJECT Design for Manufacture
→ DISPOSABLE DEVICE PROJECT Design for Manufacture
→ OUTCOME Design History File
→ MANUFACTURING Injection Molding
Physio-Assist:
A French innovative company search a partner for equipment and disposable device development.
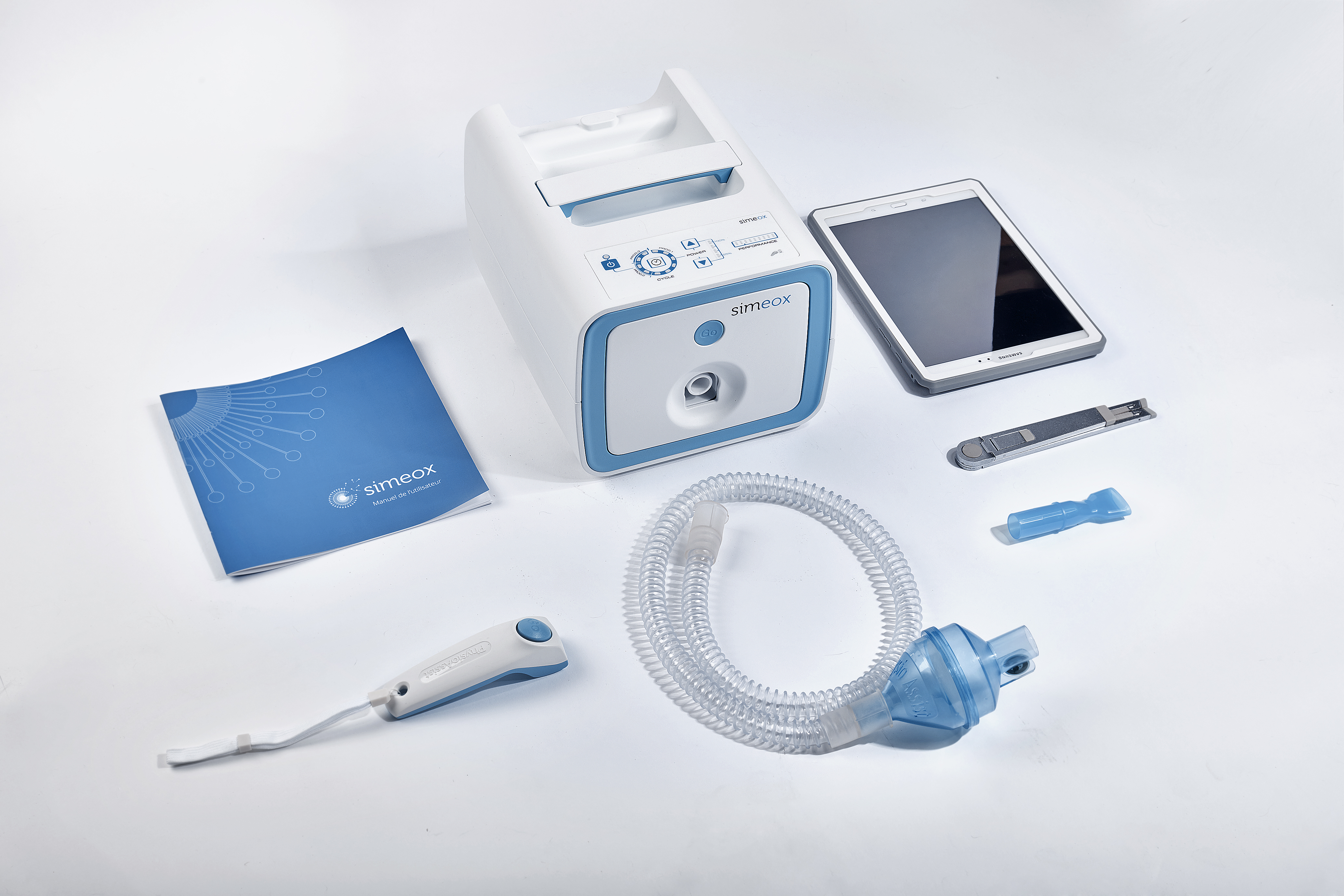
Mold Manufacturing
FMEA Risk Analysis
Material Selection
Mold and Assembling Tools Manufacturing
FMEA Risk Analysis
IQ OQ PQ Validation Plan
Performance Validation
CE Mark – OBL Contract
Ultrasonic Welding
Assembling; Packaging
After industrialization of an initial version of the device, our R&D team has fully redesigned the new version based on competitive review of functionalities. In addition, we have co-developed and co-patented part of the product.
DEVELOPMENT PROCESS → PROJECT Concept Pre-study
→ OUTCOME Design History File
→ MANUFACTURING Injection Molding
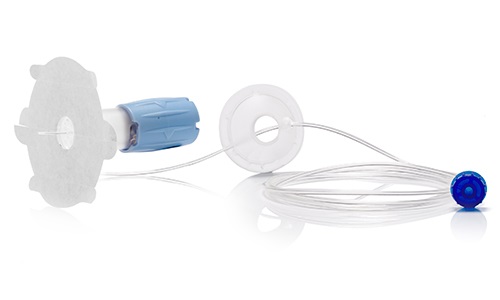
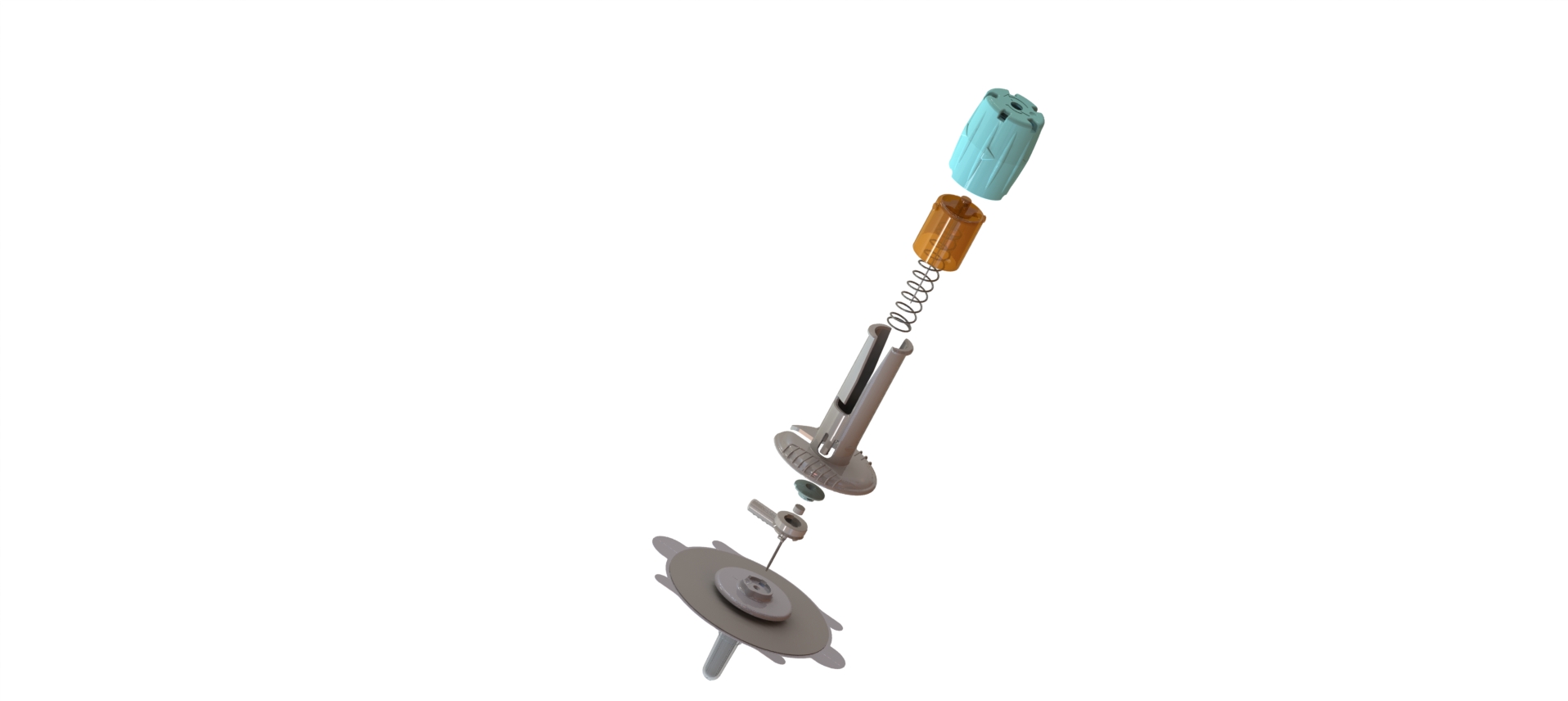
CAD Design
Rapid Prototyping
Material Selection
Design for Manufacture
Mold Manufacturing
Assembling Tools Manufacturing
FMEA Risk Analysis
IQ OQ PQ Validation Plan
CE Mark
FEP Tip Forming
Assembling
Packaging
Sterilization
For our innovative client, we have reviewed and analyzed decompression devices on the market and redefined the market standard with new design and improved performance for end users.
DEVELOPMENT PROCESS → PROJECT CAD Design
→ OUTCOME Design History File
→ MANUFACTURING Injection Molding
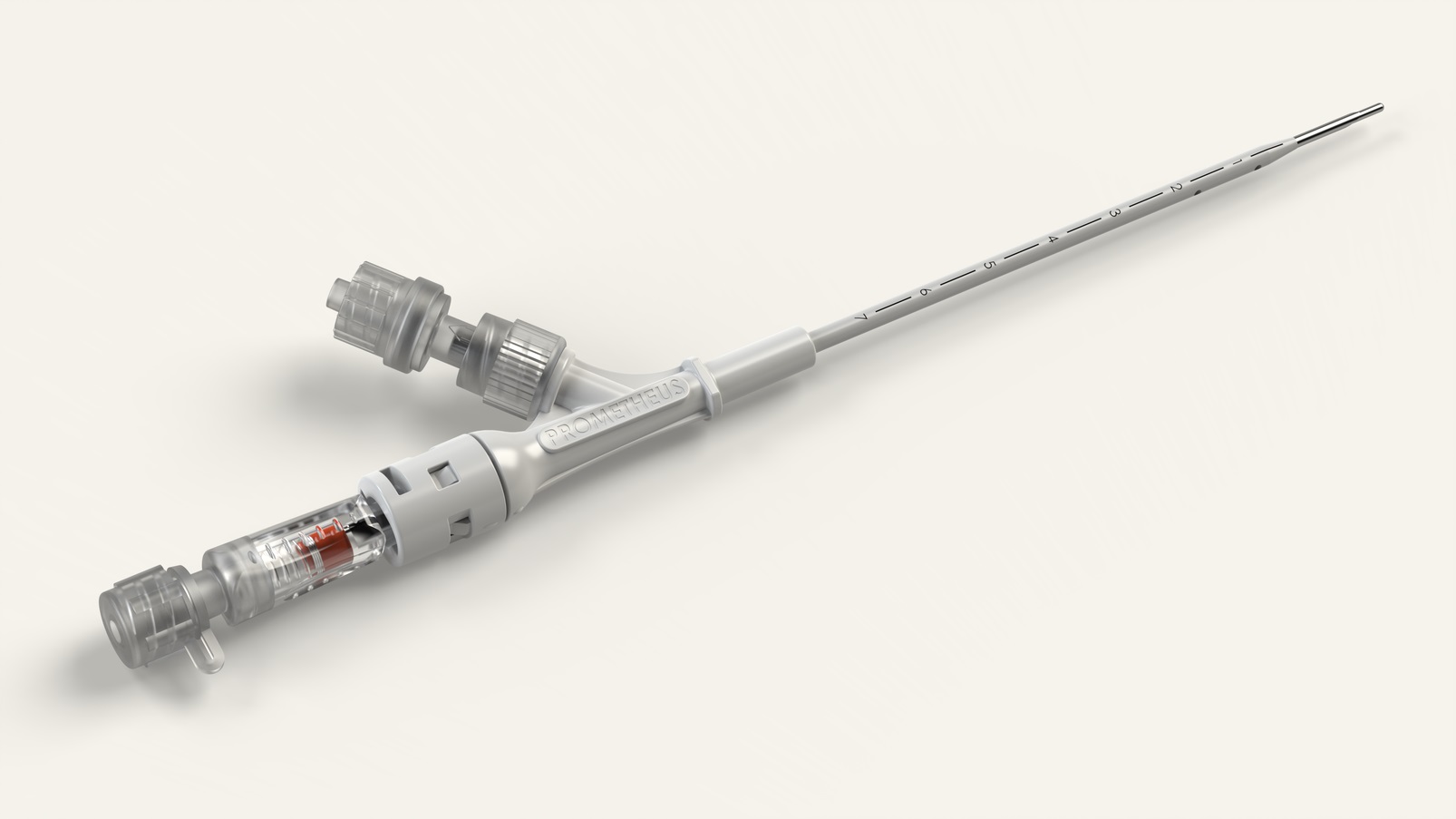
Design for Manufacture
Mold Manufacturing
Assembling Tools Manufacturing
FMEA Risk Analysis
IQ OQ PQ Validation Plan
CE Mark
Over-molding
Tip forming
Driling
Pad Printing
Assembling; Packaging; Sterilization
After the initial basic design phase, our R&D team has fully reviewed the initial prototype and help redevelop and industrialize this device to meet market requirements.
DEVELOPMENT PROCESS → PROJECT Concept Pre-study
→ OUTCOME Design History File
→ MANUFACTURING Injection Molding
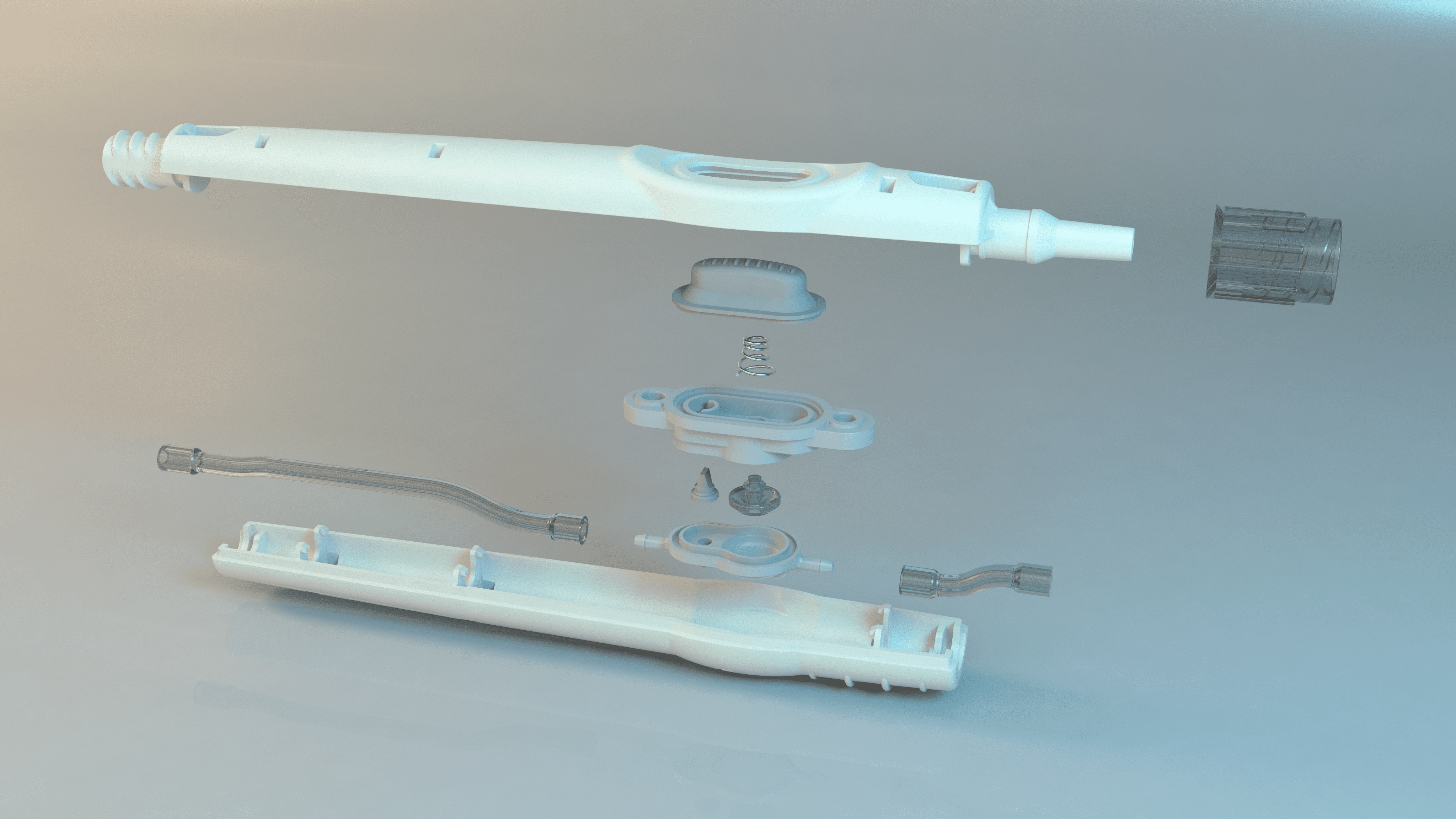
CAD Design
Rapid Prototyping
Material Selection
Design for Manufacture
Mold Manufacturing
Assembling Tools Manufacturing
FMEA Risk Analysis
IQ OQ PQ Validation Plan
Performance Validation
CE Mark – OBL Contract
Ultrasonic Welding
100% Performance testing
Assembling
Packaging
Sterilization









