The PVC used in these new flexible medical hoses is manufactured using: Consequently, the carbon footprint of this PVC is significantly lower than that of conventionally produced PVC. These new medical tubes have the same quality and properties as our conventionally produced PVC products.
Please contact us if you would like to get a quote for these medical hoses: contact@promepla.com About Medical Tubing Based in Le Bousquet d’Orb, in the French Hérault region, Medical Tubing offers its customers 30 years of experience in thermoplastic extrusion. The company serves all medical markets with tubes of all sizes, including multi-layer and multi-lumen options. It also offers compounding of multiple resins for greater responsiveness. Since 2023, Medical Tubing has been part of the Promepla Group, a full-service provider and contract manufacturer for medical devices and biopharmaceutical solutions. http://www.medical-tubing.com/
LATEST NEWS
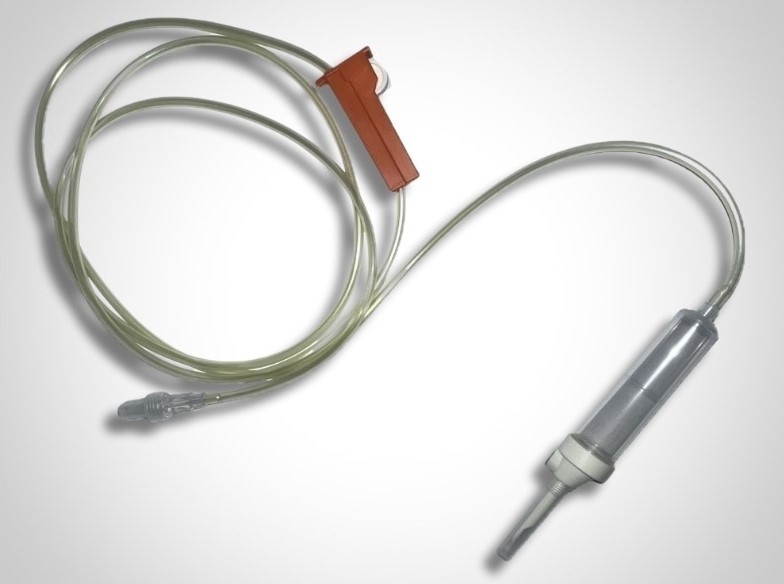
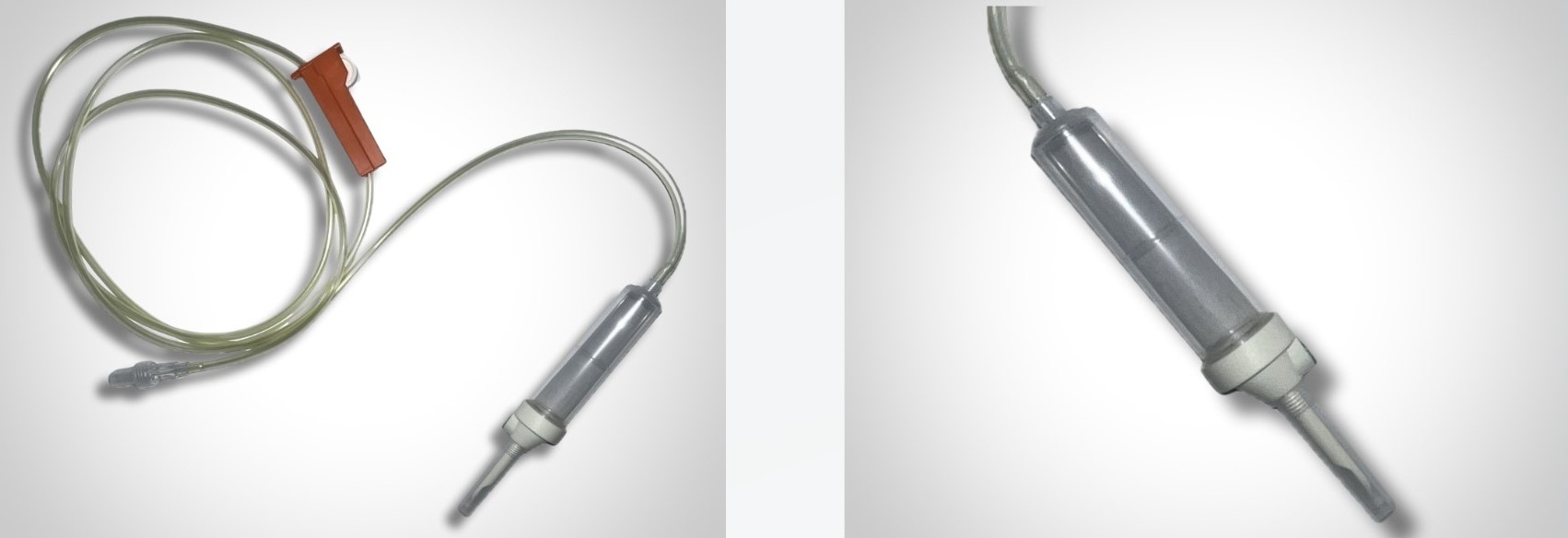
A. Hopf GmbH, a pioneer in the development of BPA-free components, now features a PVC-free IV set.
This set consists of: Regarding the various connections (since PP can be difficult to bond and requires special treatment): Do not hesitate to come back to us for more information on this PVC-free intravenous set: contact@promepla.com. About A. Hopf GmbH A. Hopf Kunststoffverarbeitung GmbH is a high-precision injection molder and mold manufacturer for medical components. Hopf products are characterized by high quality, excellence and reliability and are manufactured exclusively in Germany. Since 2022, Hopf has been part of the Promepla Group, a full-service provider and contract manufacturer for medical devices and biopharmaceutical solutions.
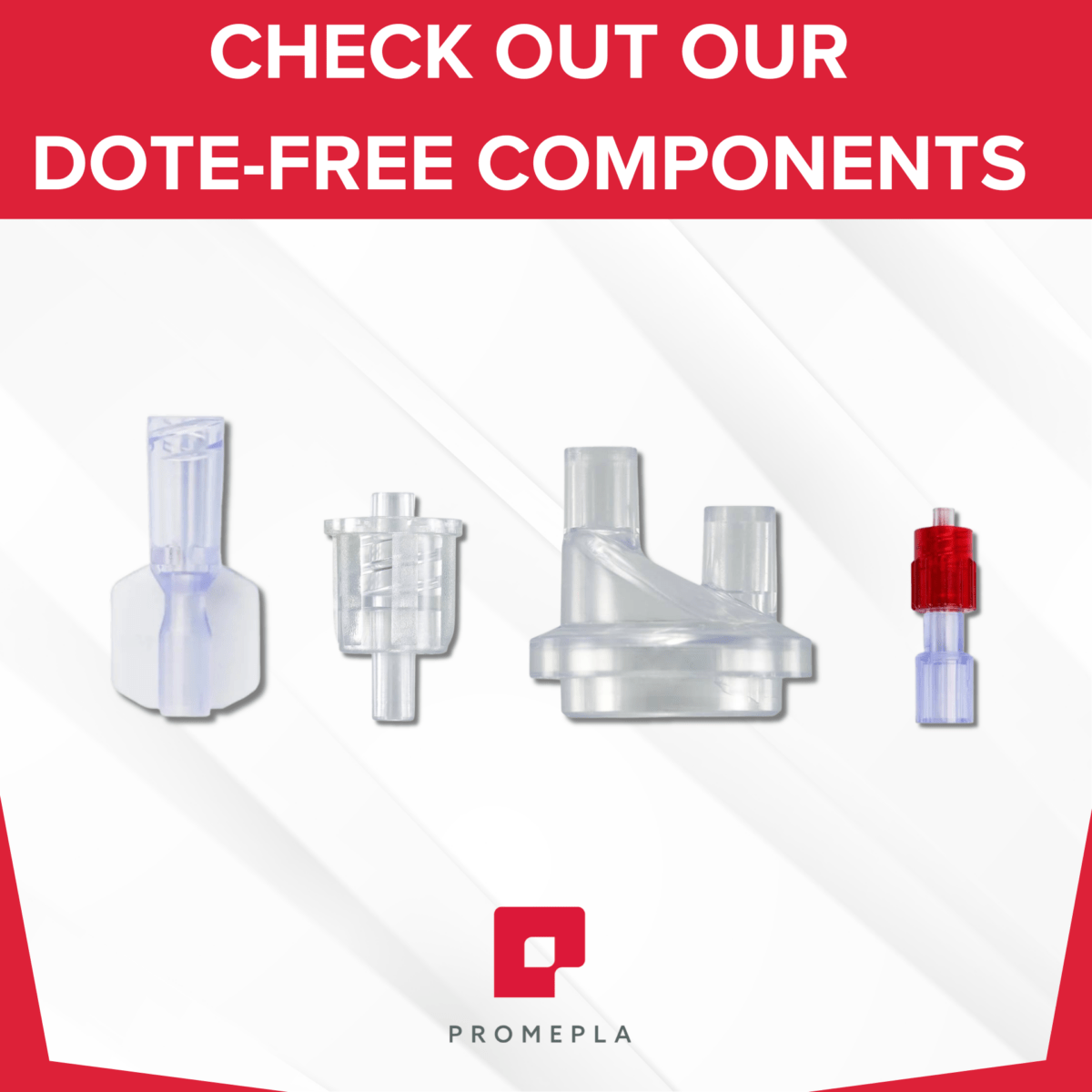
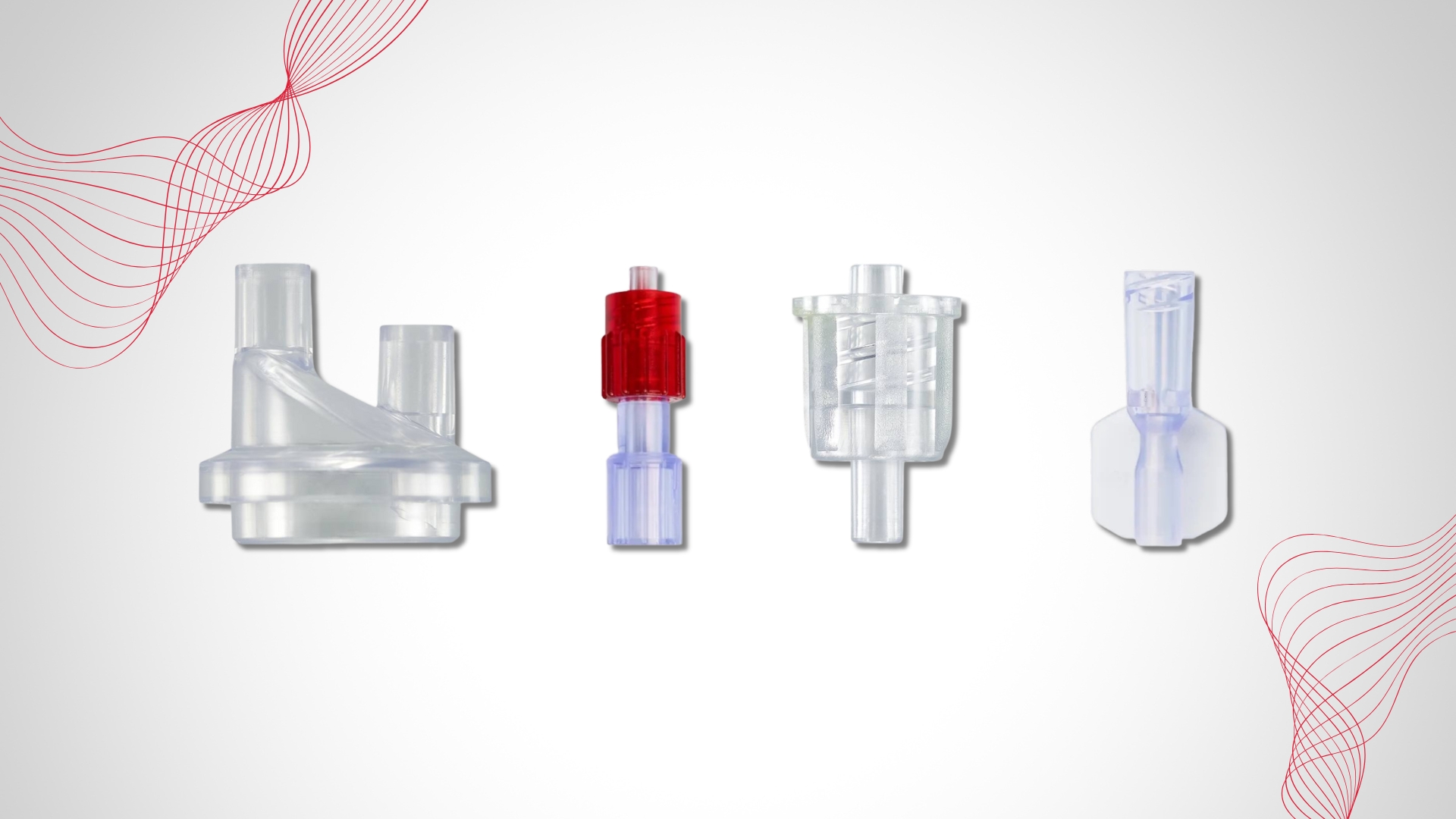
The DOTE substance (2-ethylhexyl 10-ethyl-4,4-dioctyl-7-oxo-8-oxa-3,5-dithia-4- stannatetradecanoate), a thermal stabilizer commonly used in rigid PVC, was added in 2022 to Annex XIV of the European Union (EU) REACH Authorisation List due to its “toxic for reproduction” intrinsic property (Article 57c). As a result, DOTE should no longer be used in medical device components from May 1st 2025. Do any of your PVC products still contain DOTE?
We are at your disposal to help you source alternative DOTE-free components for medical devices, do not hesitate to contact us: contact@promepla.com.
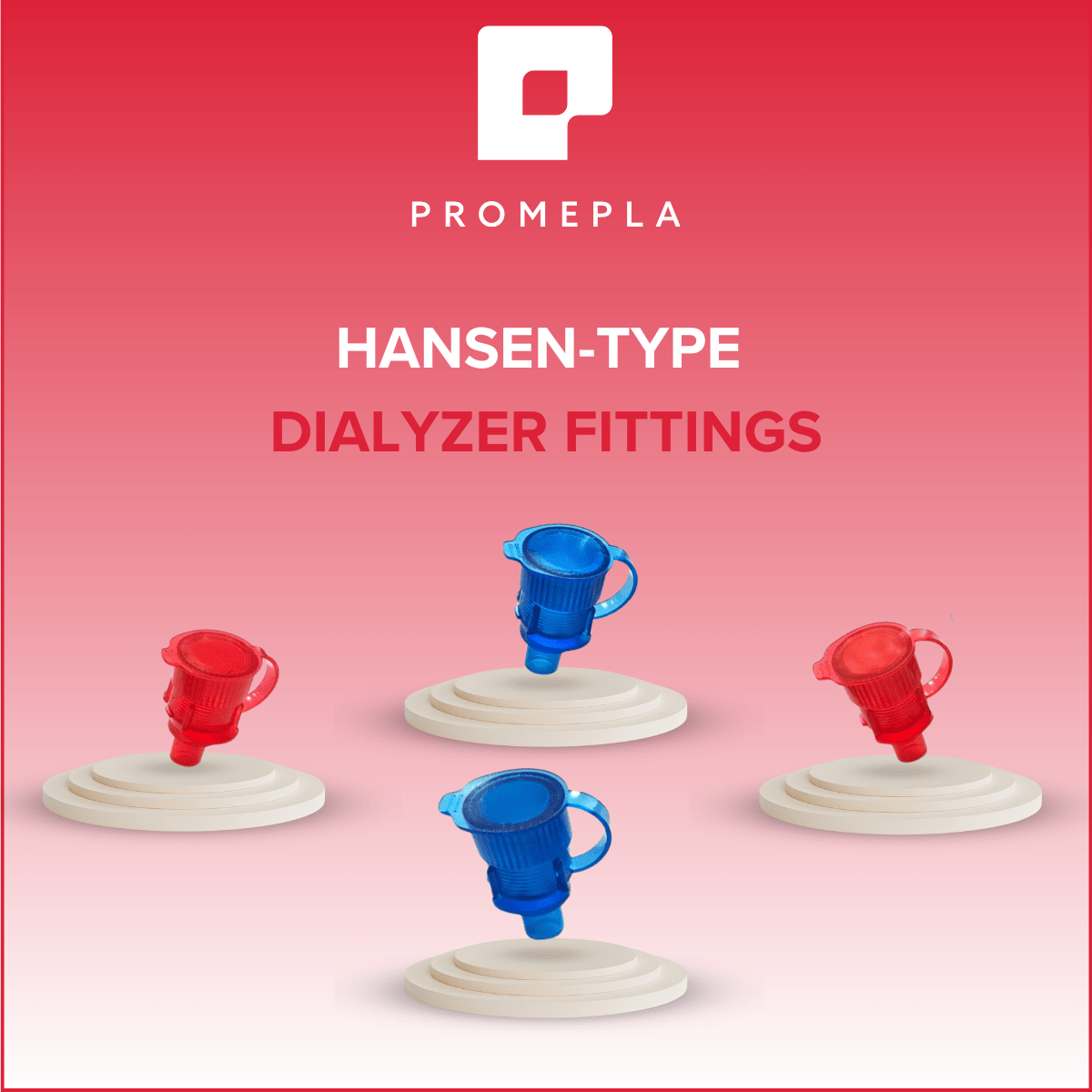
Are you looking for a coloured Hansen-type dialyzer connector? We have red and blue references back in stock!
To order them or ask for samples:
We are happy to announce that we have renewed our ISO 13485:2016 certification for 3 years. This is the result of the hard work and daily commitment of our teams.

We are expanding our range of services and industrial capabilities with the acquisition of the French company Medical Tubing.
Based in Le Bousquet d’Orb, in the Hérault region, Medical Tubing offers its customers 30 years of experience in thermoplastic extrusion. The company serves all medical markets with tubes of all sizes, multi-layer and multi-lumen. It also offers compounding of multiple resins for greater responsiveness. With Medical Tubing, we complete our offering as a solutions integrator for all single-use medical device and biopharmaceutical development projects. Following the acquisition in 2022 of A. Hopf GmbH, a German company based in Cadolzburg, we now cover the entire value chain for single-use life science devices, with more than 3,300 sqm of ISO 8 cleanroom space : These investments in France and Europe in the medical sector enable us to offer European and global medical device players a single point of entry for their projects, reducing development times and reducing costs and integrating the challenges of industrialisation and marketing from the outset. http://www.medical-tubing.com/
We are proud to announce the acquisition of A. Hopf Kunststoffverarbeitung GmbH, a family-owned German company based in Cadolzburg, specialising in plastic injection moulding for the life sciences sector.
Founded in 1949 by Mr Anton Hopf, A. Hopf GmbH was one of the pioneers of injection moulding in Germany. Since then, the family name has been associated with high quality and customised solutions for single-use life science components. With a 1600 sqm production area, 350 sqm of ISO 8 clean room and a 350 sqm tool shop, A. Hopf GmbH serves the whole of Europe from its base in Germany. The company offers a range of components for enteral and intravenous applications and any medtech activity (components for nutrition, high flow valves, production of specific parts in thermoplastics, precision injection moulded parts). Any thermoplastic material can be processed in Cadolzburg, with a focus on BPA-free solutions. In this way, A. Hopf enables us to integrate the injection moulded parts into your overall projects and respond to your needs.


Thanks to our unique manufacturing expertise, our R&D team has helped our client, a contract manufacturer, to review and improve a critical production process manufacturing equipment.
DEVELOPMENT PROCESS → PROJECT Manufacturing Process Analysis
→ OUTCOME Design History File
This has resulted in improved capabilities and quality of products delivered.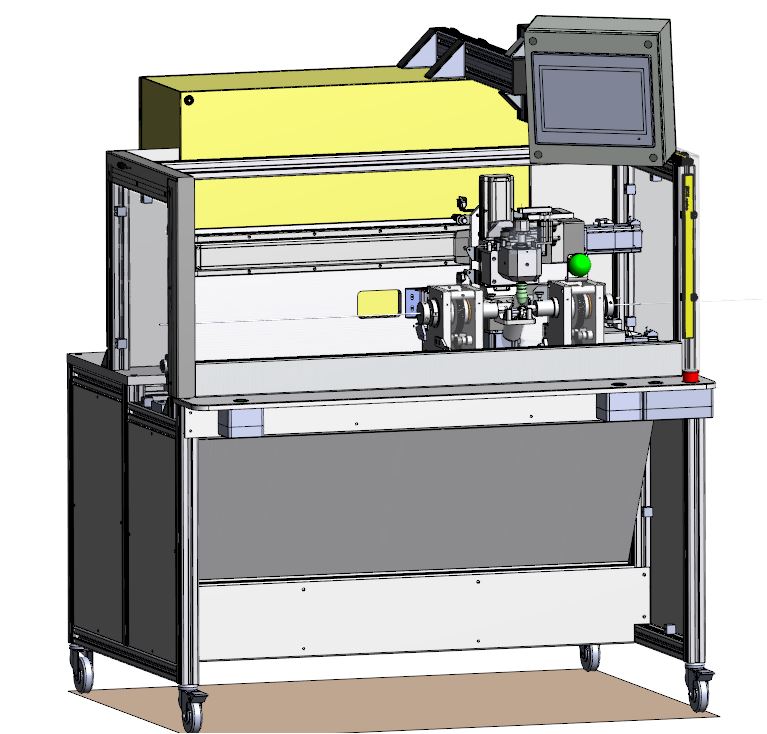
Mechanical CAD Design
Industrial Automatism Software Development
Equipment Manufacturing
FMEA Risk Analysis
IQ OQ PQ Validation Plan
CE Mark
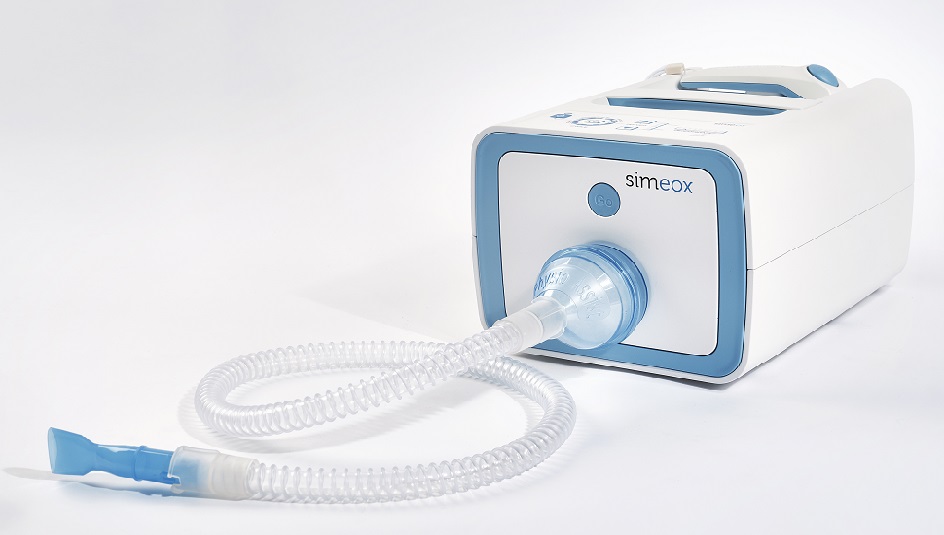
Our client, Physio-Assist has developed an innovative concept of equipment and tubing set dedicated to bronchial pathologies. We have acted as their product development and industrialization partner and have help drive the concept to fully operational equipment.
DEVELOPMENT PROCESS → EQUIPMENT PROJECT Design for Manufacture
→ DISPOSABLE DEVICE PROJECT Design for Manufacture
→ OUTCOME Design History File
→ MANUFACTURING Injection Molding
Physio-Assist:
A French innovative company search a partner for equipment and disposable device development.
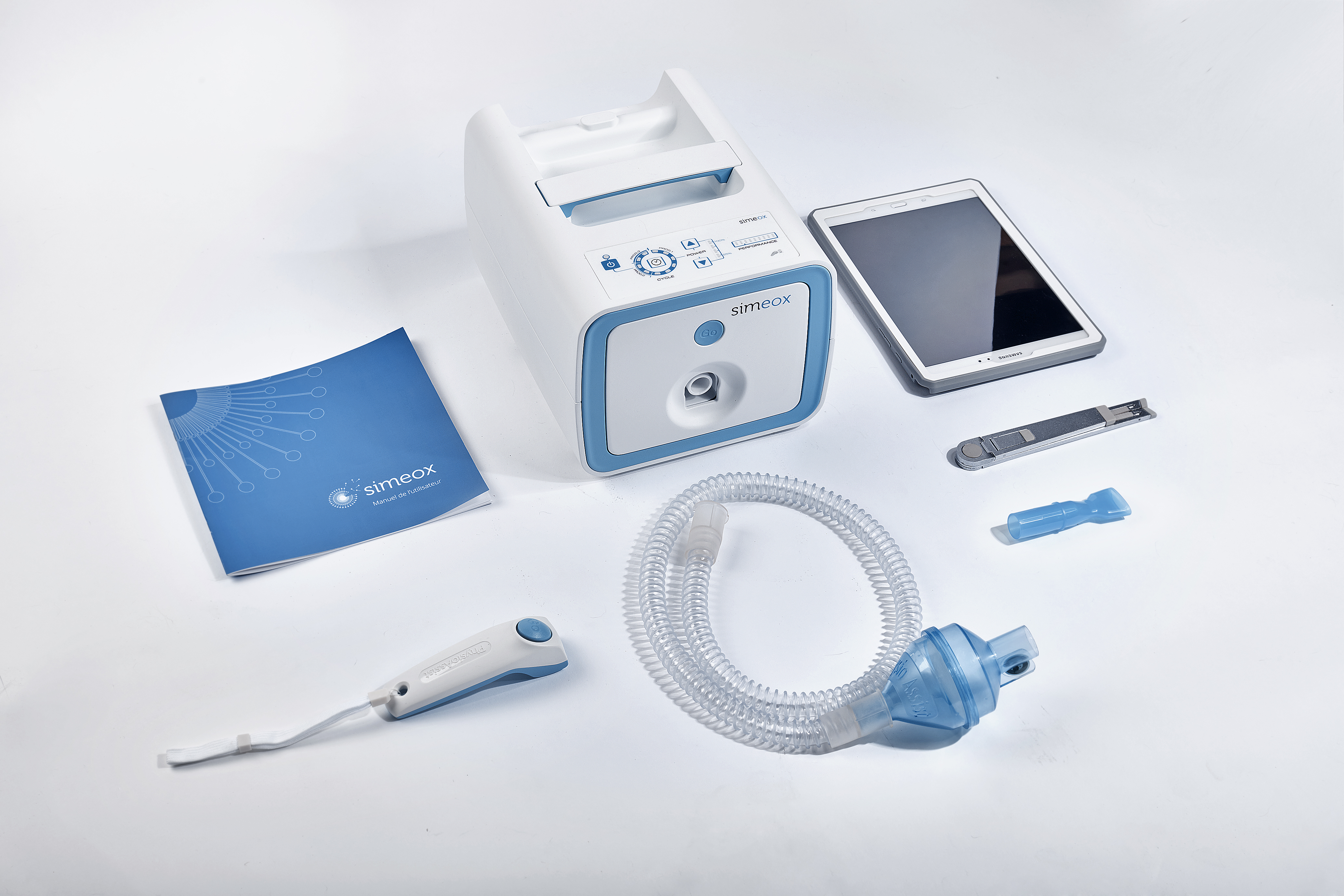
Mold Manufacturing
FMEA Risk Analysis
Material Selection
Mold and Assembling Tools Manufacturing
FMEA Risk Analysis
IQ OQ PQ Validation Plan
Performance Validation
CE Mark – OBL Contract
Ultrasonic Welding
Assembling; Packaging
After industrialization of an initial version of the device, our R&D team has fully redesigned the new version based on competitive review of functionalities. In addition, we have co-developed and co-patented part of the product.
DEVELOPMENT PROCESS → PROJECT Concept Pre-study
→ OUTCOME Design History File
→ MANUFACTURING Injection Molding
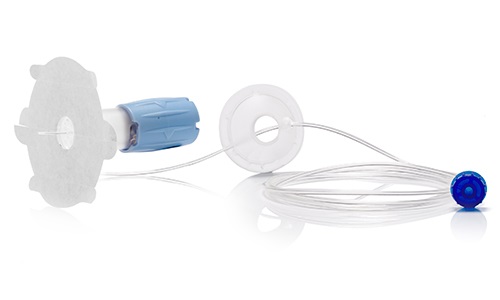
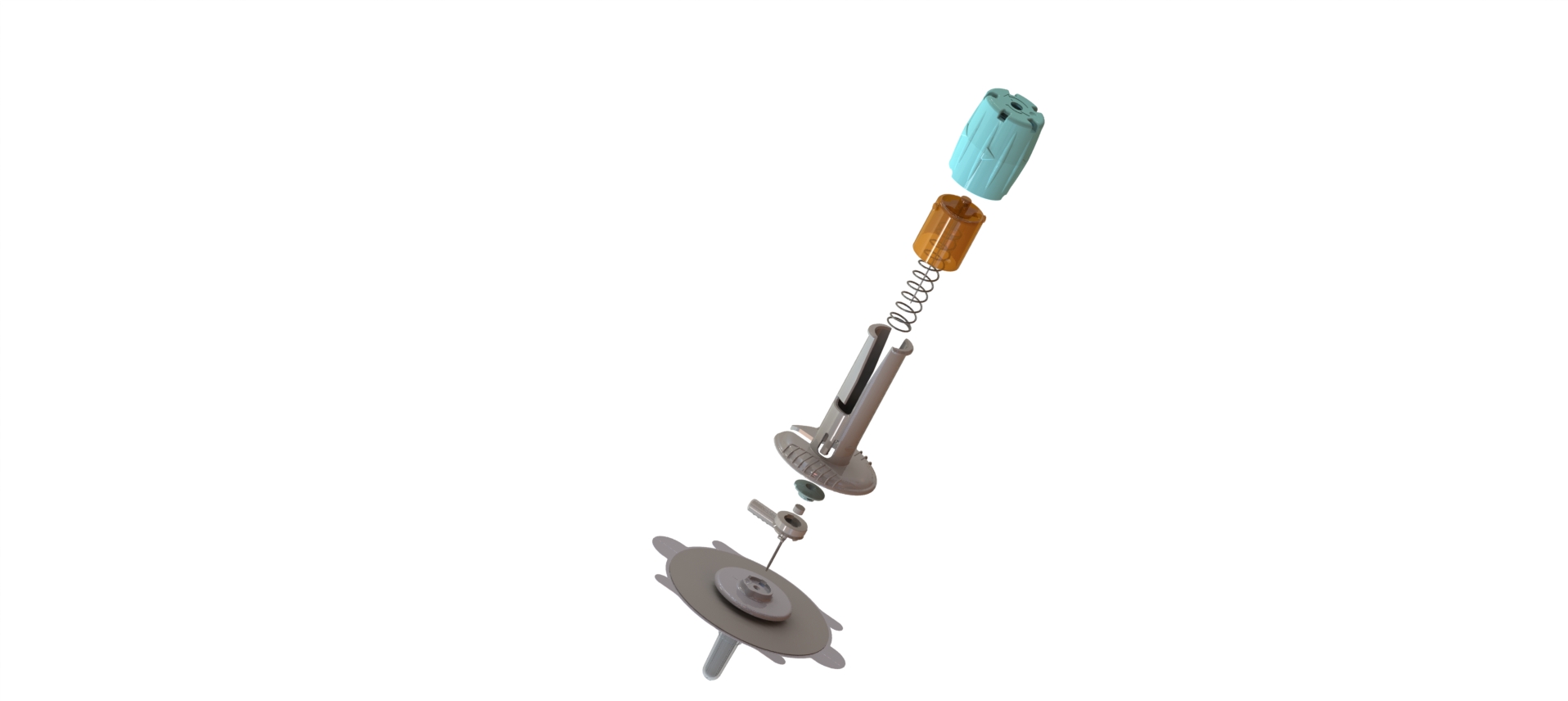
CAD Design
Rapid Prototyping
Material Selection
Design for Manufacture
Mold Manufacturing
Assembling Tools Manufacturing
FMEA Risk Analysis
IQ OQ PQ Validation Plan
CE Mark
FEP Tip Forming
Assembling
Packaging
Sterilization